Since the first reports of percutaneous catheter therapy for atrial fibrillation (AF) were published exactly twenty years ago, the field has grown exponentially. The current standard of care, which has evolved through many techniques and treatment plans, entails pulmonary vein isolation (PVI) using Radio-frequency (RF) or other energy sources. While it has been demonstrated that this treatment is superior than pharmaceutical treatment in the case of paroxysmal AF, there is a wide range of reported results.
The development of techniques over time, from electrophysiology (EP)-guided (and physically quite simple) procedures like trigger elimination inside the pulmonary veins (PVs) to more empirical creation of circular lesions around the PVs, can be partially ascribed to this variety. The latter method does away with difficulties like trigger recognition and lessens safety issues like PV stenosis, but it also presents new difficulties, most of which are connected to catheter positioning in the challenging three-dimensional (3D) region of the left atrium (LA).
The use—or rather lack of use—of basic two-dimensional (2D) fluoroscopy is one of the characteristics of catheter management in the LA. Fluoroscopy landmarks for AF ablation are few compared to traditional methods (such as right atrial or ventricular manipulation).
Additionally, accurate energy delivery at essential complex 3D structures such the transition between the left atrial appendage and the left superior PV is crucial for safe and effective PVI. To accomplish this, a number of tools have been created, ideally combining several of these helpful aspects, that either assist the operator in understanding anatomy, enable 3D catheter navigation, or reduce radiation exposure.
Rotational Angiography
Utilising single-plane radiography equipment, three-dimensional rotational angiography is a novel technique that enables the reconstruction of tomographic slices of a volume of interest similarly to computed tomography (CT). To supplement non-fluoroscopic navigation systems, the reconstructed 3D models can be utilised instead of standard CT or magnetic resonance imaging (MRI), or they can be superimposed over 2D fluoroscopic pictures. Contrary to CT/MRI, this method enables ongoing ablation procedures to be imaged in near real-time in 3D, preventing volume mismatch brought on by changes in volume status, rhythm, or even table shape (influencing chest geometry). The advantages of 3DRA versus pre-procedural imaging go beyond accuracy. Additionally, it offers significant advantages in terms of logistics and financial efficiency.
Three-dimensional Rotational Angiography as Electroanatomical Mapping Substitute
Due to the challenges in 3D navigation of the LA, Electroanatomical mapping (EAM) has quickly evolved to become the standard for guiding RF ablation. By moving a catheter through the space of any geometry, EAM enables a user to build any geometry. The obvious negative is that at the start of the mapping phase, all geometry is “unknown” an operator must learn geometry as they go along. This means that a high level of operator proficiency is needed, and there is a risk of “missing” key anatomical components – particularly in cases of variable anatomy like the presence of a roof vein or other congenital malformations.
Additionally, some single-shot technologies for PVI, such as balloon cryotherapy and multipolar RF, are incompatible with EAM systems. These settings completely lack 3D LA imaging; instead, the positioning of these devices relies on assumptions about the shape of the PV antrum, for which special catheters are needed, and typically requires 2D contrast injections to ensure proper placement.
3DRA models can be superimposed over live fluoroscopy as an alternative to an EAM workflow or as a supplement to a single-shot device workflow. Both the 3DRA segmentation workstation and the fluoroscopy apparatus are connected to one another and have a means of communication. As a result, the fluoroscopy system can display a 3D model that is suitably scaled and rotated for any live 2D fluoroscopy angle that the operator selects.
Recent improvements have made it possible to integrate activation and substrate maps into the 2D/3D model by connecting an EP recording device to the setup. Modern systems allow the marking of places of interest such ablation sites for tracking. A purely 3DRA-based workflow has limitations compared with EAM, mainly due to the inherent 2D nature of the live fluoroscopy image and the physical constraints within which a fluoroscopy gantry needs to operate.
Importantly, only continuous fluoroscopy exposure makes catheter visualisation possible. However, from a cost viewpoint, significant gains are possible because no substantial disposable costs are incurred; consequently, in carefully chosen clinical circumstances, a 3DRA model as an EAM substitute may make very good sense.
Three-dimensional Rotational Angiography and Electroanatomical Mapping Integration
Further integration of imaging systems has led to the linking of existing fluoroscopy systems to EAM systems. This enables a 3D display to be fused with 2D X-ray backdrops that correspond to a specific orientation on an EAM system (Fig). The intuitive application of such a feature is catheter positioning with minimal use of fluoroscopy. The EAM system and fluoroscopy now share a single coordinate system, which is more significant. This removes any experience barrier and the possibility of “missing” sections of anatomy because a coupled fluoro & EAM system can display a 3DRA-derived volume in its correct position without needing to collect even a single anatomy point from the catheter.
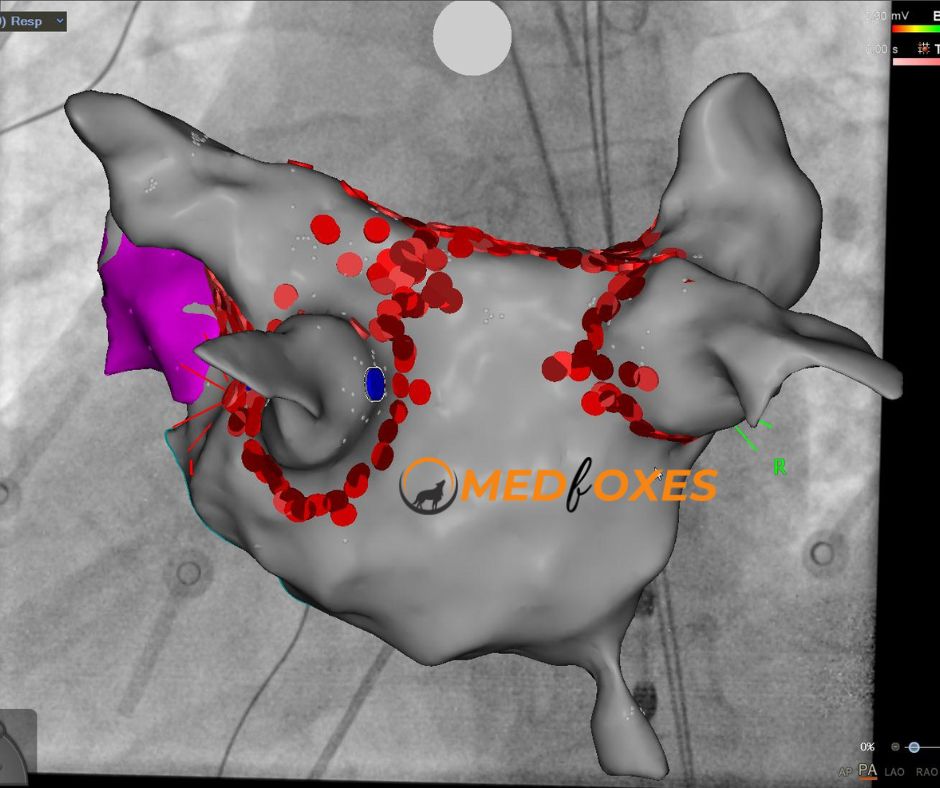
The advantages of EAM (non-fluoroscopic catheter navigation) and 3DRA (full and precise, CT-like anatomy) can be used to create a highly reproducible process for PVI that may be able to cut down on procedure time and radiation exposure.
Conclusion
Technological advances have evolved the field of AF ablation – an anatomically challenging procedure is increasingly supported by advanced imaging solutions. By combining various systems, these solutions are increasingly deployed in a synergistic manner. To improve procedural safety and efficacy during ablation, a number of well-established and cutting-edge methods work to present an intuitive and maximally accurate picture of the LA and its particular anatomic regions. One of these, 3DRA, is an imaging modality that may currently be used in high-volume clinical settings, either as a stand-alone tool or when connected with 3D navigation systems, the latter of which enables PVI processes with almost little radiation.
Reference
EP Europace, Volume 19, Issue 8, August 2017, Pages 1310–1316,
