QFR and OFR in the Assessment of intermediate CAD that may benefit from percutaneous coronary intervention, a physiological lesion assessment is indicated. The optical flow ratio (OFR) is a more modern approach for the quick and automated assessment of coronary physiology from intracoronary optical coherence tomography (OCT). The quantitative flow ratio (QFR) was created to derive coronary physiology from angiographic Images.
Indication and Use of Physiologic Lesion Assessment
Quantitative Flow Ratio
The accuracy of coronary geometry reconstruction and hemodynamic modelling are the foundations of FFR computation. 3D quantitative coronary angiography (3D-QCA) uses a minimum of two angiographic acquisitions, usually separated by 25°, to rebuild the coronary geometry.
After the conventional views were taken, the rotational angiography images were taken while the catheter was still engaged in the coronary artery. Iso-centering the patient was essential for proper alignment for the spin acquisition. This was accomplished by focusing the area of interest in both the anteroposterior and lateral projections of the fluoroscopic field.
Following Iso-centering, a test run in the spin trajectory was done at normal speed without fluoroscopy to check that gantry motion was not obstructed. The rotation was started as soon as the contrast had filled the entire coronary artery. The gantry moved through an arc at a rate of 40° during spin acquisition. After the contrast had totally vanished from the coronary artery, cine acquisition was ended. For all spin acquisitions, manual injection was used. The LCA spins were acquired by rotating the gantry from 30 degrees RAO 30 degrees cranial to 90 degrees LAO 30 degrees cranial. The RCA spins began at 60 degrees LAO and were recorded at 30 degrees RAO.
The 3D QCA was performed using the automated reconstruction system

Optical Coherence Tomography-derived Fractional Flow Reserve
OCT can visualize vessel structure at a considerably greater resolution than ICA and overcome the limitations of Invasive Coronary Angiography, such as vessel overlap and foreshortening. As a result, for the derivation of OFR, a computational approach such as QFR was applied to OCT pictures. The lumen contours are automatically identified and overlaid in 3D once an OCT pullback is acquired according to standard clinical procedure.
To quantify the side-branch areas, the cut planes of the side-branches ostia are recreated. The latter enables precise derivation of the step-down reference lumen as if there were no stenosis. Finally, the volumetric flow rate is calculated by multiplying a fixed flow rate of 0.35 m/s by the reference lumen size, and the OFR is computed for each cross-section and superimposed on the OCT images. The computation of OFR is a commercially available service. A recent study found that OFR outperformed QFR in terms of diagnostic performance, owing to the integration of side-branches and correct OCT-derived lumen dimensions.
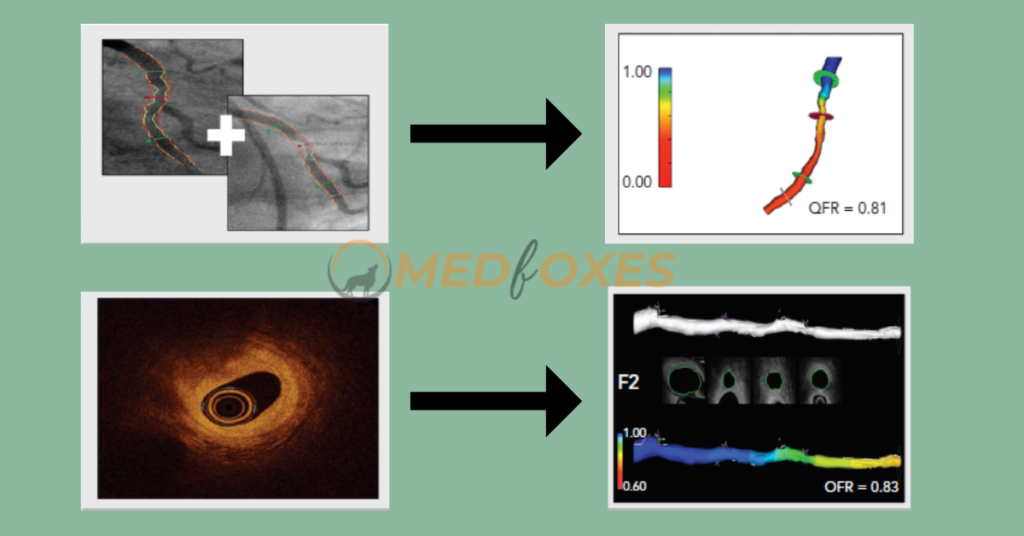
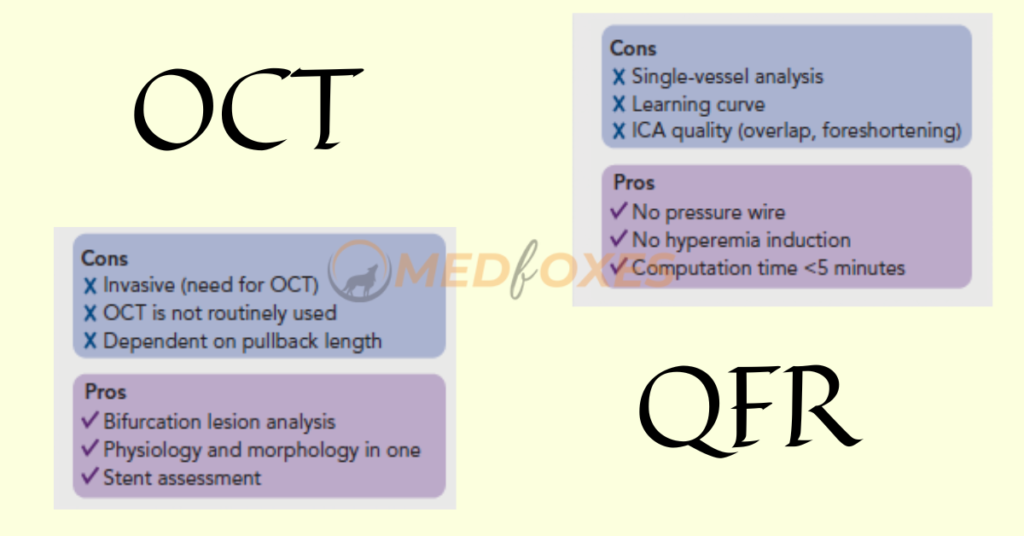
Identification of Coronary Artery Stenosis with Indication for Revascularization
An FFR of less than 0.80 is commonly considered as a good predictor of vessels that will benefit from revascularization. Therefore, FFR was routinely used as a reference standard in diagnostic studies evaluating new physiologic indices. In retrospective, prospective offline, and prospective in procedural investigations, QFR and OFR exhibit strong diagnostic and numerical agreement with FFR.

The diagnostic accuracy of in-procedure QFR, using the FFR as a reference standard, was shown to be high. Importantly, QFR was determined in the same amount of time as traditional FFR measures. Close to the FFR 0.80 cut-off point, diagnostic certainty declines, and the usefulness of revascularization is questioned.
Patients with chronic kidney disease, diabetes, prior MI, microcirculatory dysfunction, severe stenosis (high percentage diameter stenosis or lengthy lesion length), and severe aortic stenosis have higher discordance between QFR and FFR, according to observational data. The overall validation results, however, appear to be comparable and promising.
Virtual Percutaneous Intervention Planning
3D-QCA offers detailed vessel dimensions to the operator, similar to lumen dimensions determined by OCT. This information is useful for treatment planning. Furthermore, QFR software allows for the computation of residual QFR, which can be used to forecast the effect of stenting individual lesions in circumstances when there are multiple lesions or the disease is broad. The current application, however, has inadequate data.
Study found a correlation between pre-PCI residual QFR (virtual PCI) and the actual post-PCI FFR results. Of note, such a correlation might be reduced by the presence of in-stent pressure drop as result of suboptimal PCI. Virtual PCI, on the other hand, presupposes that the target vessel segment has been entirely revascularized. Furthermore, QFR and OFR provide a pressure pullback, similar to FFR, which can be useful in distinguishing localised disease from widespread disease.
With motorised FFR pullbacks, the pullback pressure gradient was recently introduced to identify arteriosclerotic disease patterns. By recognising diffuse illness that can frequently be handled with effective medical therapy or a coronary artery bypass graft, this could allow for more personalised patient decision making. Given the similarity of QFR and OFR to FFR, virtual pullbacks formed from QFR and OFR may be suited for this purpose as well, and will be the topic of future research.
Conclusion
By giving precise coronary anatomy and imaging-derived physiology, QFR and OFR have the potential to improve clinical practice. The integration of anatomy and physiology could help doctors make better treatment decisions and, if necessary, maximize revascularization. To verify the efficacy of these solutions when used by users outside of highly qualified core laboratories, the methodologies must be validated by ongoing randomised outcome trials.
References:
International J Cardiovasc Imaging 2012; 28(7): 1627–1634.
Published online 2011 Dec 18. DOI: 10.1007/s10554-011-9993-0
US Cardiology Review 2020;14:e09. DOI: https://doi.org/10.15420/usc.2020.09

